Yeah, yeah, yeah, we all know that OSHA and infection control are crucial pillars of what we do each and every day in dentistry. It is federally mandated (enforced by law) that employers provide OSHA training when an employee starts the job and at least yearly thereafter.1 In some states, continuing education courses in infection control are required for re-licensure, too. Through the fast pace of our profession, sometimes, the small details may be lost.
Here are 11 OSHA and infection control details that dental professionals don’t want to overlook.
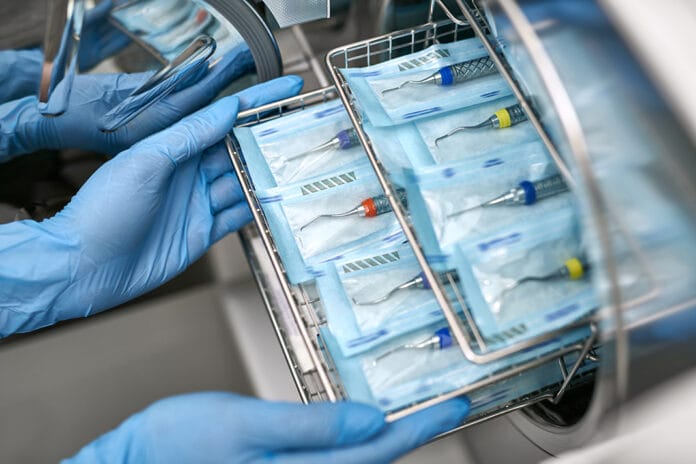
1) Understand your sterilization equipment
The sterilization process is not a one-size, or in this case, one cycle fits all. Each autoclave, ultrasonic bath, and everything in between varies based on the manufacturer, model, or version. Some autoclaves require more time to sterilize than others. In whatever setting you work in, consult the specific manufacturer’s instructions and sterilization processes.
According to the Centers for Disease Control and Prevention (CDC), manufacturer’s instructions should be readily available.2 You can also look up the manufacturer name and model of the equipment to easily find the user’s manual. Be sure to discern how to sterilize instrument pouches (paper or plastic side up) according to the manufacturer’s instructions.
Regularly monitor sterilization equipment, such as autoclaves, to ensure they are functioning properly. This may include weekly biological spore testing. The CDC recommends that “sterilization is best monitored using a combination of mechanical, chemical, and biological indicators.”2
2) Ensure you are wrapping and bagging instruments correctly
First, you need to be safe through this process. When bagging instruments, it is important to use sterilization pouches that are properly sized and not too small. If you are bagging multiple instruments and are struggling to stuff them all in, size up. You can use a smaller bag if it’s only an instrument or two.3
Don’t forget to not over-fold your sterilization bag past the indicated fold line when sealing. In addition, make sure the bag is not punctured before or after the sterilization process.3
If wrapping instruments, ensure all instruments are put into place and not poking out before closing your cassette. This is not only a safety issue; instruments not properly placed can also pierce the wrap.4
If there is any damage or puncture to the wrap or bag or whatever you are using, instruments cannot be used and need to be re-sterilized.3,4
3) Transport contaminated instruments to sterilization safely
According to the Occupational Safety and Health Administration (OSHA), contaminated instruments must be safely transported to the sterilization area. OSHA states that instrument transport should occur “immediately or as soon as possible after use.” Additionally, OSHA states the instruments must be transported in a container that is puncture-resistant and leakproof on all sides and bottom.5
During transport, full personal protective equipment should be worn. This is not only safe for you but those around you. This avoids injury if you are accidentally bumped into.5
OSHA notes that if these containers (i.e., contaminated instruments, sharps containers) are leaving the facility, they need to be color-coded (fluorescent orange or orange-red )or labeled with a biohazard icon.5
4) Properly clean and disinfect non-critical environmental surfaces
The CDC categorizes patient care items based on their potential risk of transmission of infection when used. The categories include non-critical, semi-critical, and critical. Non-critical environmental surfaces are further categorized as housekeeping and clinical contact surfaces.2
Clinical contact surfaces can be contaminated by spatter, spray, or aerosols generated during patient care or by clinician’s hands during treatment. If disposable barriers are not used, clinical contact surfaces should be cleaned and disinfected between patients with an Environmental Protection Agency (EPA)-registered hospital disinfectant with an HIV or HBV claim (low-level disinfection) or if visibly soiled an intermediate-level (i.e., tuberculocidal claim) disinfectant should be used.2
Keep in mind there is a difference between cleaning and disinfecting your dental unit – both must be done. There’s a reason for that. Cleaning involves physically removing blood and inorganic and organic matter that interferes with the germicidal activity of a disinfectant. Wipe surfaces at least twice: one to “dust and clean” and the second to “disinfect.” The CDC states, “If a surface is not cleaned first, the success of the disinfection process can be compromised.”2
Clinical contact items that you might use disposable barriers include pens, the radiograph unit, or dental unit handles. If visibly contaminated with blood or other potentially infectious materials, an intermediate-level disinfectant should be used.2
Non-critical housekeeping surfaces include floors, walls, and sinks. These items have less risk of disease transmission and can be decontaminated with less vigorous methods compared to clinical contact and patient care items.2
Since the COVID-19 pandemic, many people have become familiar with what we refer to as non-critical environmental surfaces, but as a dental professional, take a step back and see what not only you or the people you work with touch but also what the patients touch. For example, if a patient points to a tooth that is bothering them in their mouth, what else do they touch afterward? Anything they touch could be contaminated because they put their fingers in their mouth.
5) Know the details about the disinfection products you use
Like sterilization, the products you clean and disinfect your operatory are also not one-size-fits-all. Look at specific properties such as kill time (amount of contact time to disinfect properly). Certain products require more contact time than others to properly disinfect and kill TB, bacteria, fungi, and viruses.6
Keep in mind to avoid mixing or combining disinfectant products when using sprays and wipes together. Mixing different disinfectants can create fumes that could be dangerous to breathe in.7
6) Sterilize handpieces in between patients
Though slow-speed hygiene handpieces are considered semi-critical, according to the CDC, handpieces that attach to dental unit air or waterlines should be sterilized in between patients. The internal motor can become contaminated by retracting oral fluids, then expelled during later uses, so surface disinfection alone is not acceptable.2,8
Do not forget to lubricate handpieces if the manufacturer’s instructions state they require it.2 You can use either a maintenance unit that automatically lubricates your handpiece or lubricate by hand.
7) Maintain eyewash stations
Eyewash stations are required in workplaces that use corrosive chemicals, in HBV and HIV research facilities, and in workplaces that pose a risk of eyes being splashed with 0.1% or greater formaldehyde or in any workplace where materials may cause injury or infection of the eyes.9
In addition to providing an eyewash station, OSHA recommends proper maintenance to reduce the risk of contamination with organisms such as Acanthamoeba, Pseudomonas, and Legionella. If eyewash stations are not properly maintained, they can do more harm than good through the organisms coming in contact with an injured eye or inhaling organisms that could potentially cause a serious illness. Refer to the manufacturer’s instructions for proper maintenance.9
8) Don’t forget waterlines and traps
The CDC gives a great explanation of why it is crucial to maintain your waterlines properly. They state that biofilm and bacteria commonly occur in “dental unit waterlines because of the long, small-diameter tubing and low flow rates used in dentistry, the frequent periods of stagnation, and the potential for retraction of oral fluids.”10
OSHA has multiple guidelines in terms of waterline maintenance. First, the water source needs to meet the Environmental Protection Agency (EPA) drinking water standards. You should also review the manufacturer’s recommendations for your specific dental unit and equipment. “Tablet systems, continuous release straws and cartridges, initial and periodic shock treatments, and centralized systems” are common waterline treatment products.10,11
Dental traps should be regularly replaced if they are disposable. If not replaced, you may notice your suctions not working as well. When suction is not properly functioning and is less powerful, this could then lead to poorly controlled aerosols and splatter. Studies show the need for > 250 L/min airflow rate to ensure proper function to reduce aerosols. Smaller line diameter affects the airflow rate, reducing efficacy. As you can imagine, lines that are clogged with biofilm and debris will be narrower and reduce airflow rate.12 Check the manufacturer manual for specific guidance on your unit’s trap.
9) Avoid backflow!
This is a huge one for me, and a very common oversite providers forget about. “Kissing Mr. Thirsty” may be causing possible harm to your patients. Having patients close their lips around the suction changes the pressure in the suction line and results in the risk of “suck back” or backflow.2
There are many products on the market to reduce the risk of backflow that can be easily implemented and added to suction, such as a saliva ejector adapter. On a positive note, I have noticed more continuing education courses focusing on waterline maintenance and backflow avoidance.
10) Ensure proper disposal and safety of sharps
Used local anesthesia carpules and needles should be disposed of in the proper container. According to OSHA, unbroken carpules are not required to be disposed of in a sharps container, but you may also need to consider your state’s requirements and regulations.5 Sharps containers should be labeled correctly, readily available, and as close to the area where sharps are being used. Do not dispose of sharps in a full sharps container to avoid self-injury.5
Speaking of needles, double-check how you handle your syringe and needle before and after injecting. Are you recapping safely? OSHA recommends using the scooping technique or a recapping device, of which many different types are available. Do not use two hands to recap a needle.5
11) Are your first aid and medical kits ready if there’s a medical emergency?
Next time you are in your office or workplace, take a look at your first aid and medical kits. Are there expired items in there? Is everything stocked up? If there was a medical emergency, are the required items present in the kits?
OSHA does not specify what needs to be included in a first aid kit, as this can be state-specific. OSHA states, “Employers should assess the specific needs of their worksite periodically and augment the first aid kit appropriately.”1,13
However, the American Dental Association (ADA) has created some recommendations to help. For basic emergency kits, the ADA recommends:1
- Portable oxygen cylinder (E size) with regulator
- Supplemental oxygen delivery devices:
- Nasal cannula
- Nonrebreathing mask with oxygen reservoir
- Nasal hood
- Bag-valve-mask device with oxygen reservoir
- Oropharyngeal airways (adult sizes 7, 8, and 9 CM)
- Magill forceps
- Automated external defibrillator (AED)
- Stethoscope
- Sphygmomanometer with adult small, medium, and large cuff sizes
- Wall clocks with second-hand
- Epinephrine
- Diphenhydramine (histamine blocker)
- Nitroglycerin
- Bronchodilator (i.e., albuterol)
- Glucose
- Aspirin
In addition, disposable razors are a good idea to keep handy next to the AED. Considering the opioid crisis, including naloxone (Narcan) in your medical emergency kit might be something your office wants to consider.
In Closing
Each office, clinic, or workplace is different, but the quality of infection control should be the same. It is part of the standard of care we first learned about in dental hygiene school. The goal and pledge is to do no harm, so don’t let these small details get lost.
Before you leave, check out the Today’s RDH self-study CE courses. All courses are peer-reviewed and non-sponsored to focus solely on high-quality education. Click here now.
Listen to the Today’s RDH Dental Hygiene Podcast Below:
References
- Department of Scientific Information, Evidence Synthesis and Translation Research, ADA Science and Research Institute, LLC. (2022, October 25). Occupational Safety and Health Administration (OSHA). American Dental Association. https://www.ada.org/en/resources/research/science-and-research-institute/oral-health-topics/occupational-safety-and-health-administration
- Kohn, W.G., Collins, A.S., Cleveland, J.L., et al. Guidelines for Infection Control in Dental Health-care Settings – 2003 [MMWR Report No. 17]. Centers for Disease Control and Prevention. 2003; 52: No. RR-17. http://www.cdc.gov/mmwr/PDF/rr/rr5217.pdf
- It’s a Wrap! Everything You Need to Know About Sterilization Pouches. (2021, July 16). Medicom. https://medicom.com/blog/its-a-wrap-everything-you-need-to-know-about-sterilization-pouches/
- Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities. (2017). Association for the Advancement of Medical Instrumentation. https://array.aami.org/doi/book/10.2345/9781570208027
- Bloodborne Pathogens. (n.d.). Occupational Safety and Health Administration. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030
- Selected EPA-Registered Disinfectants. (2023, December 7). United States Environmental Protection Agency. https://www.epa.gov/pesticide-registration/selected-epa-registered-disinfectants
- Guidance for Cleaning and Disinfecting: Public Spaces, Workplaces, Businesses, Schools, and Homes. (2020, April 28). United States Environmental Protection Agency. https://www.epa.gov/sites/default/files/2020-04/documents/316485-c_reopeningamerica_guidance_4.19_6pm.pdf
- Dental Handpieces and Other Devices Attached to Air and Waterlines. (2016, March 25). Centers for Disease Control and Prevention. https://www.cdc.gov/oralhealth/infectioncontrol/faqs/dental-handpieces.html
- Health Effects from Contaminated Water in Eyewash Stations. (2015). Occupational Safety and Health Administration. https://www.osha.gov/sites/default/files/publications/OSHA3818.pdf
- Dental Unit Water Quality. (2016, March 25). Centers for Disease Control and Prevention. https://www.cdc.gov/oralhealth/infectioncontrol/faqs/dental-unit-water-quality.html
- The Importance of Dental Unit Waterline Maintenance – Infection Control. (2017, February 22). OSHA Review Incorporated. https://oshareview.com/2017/02/the-importance-of-dental-unit-waterline-maintenance-infection-control/
- Graetz, C., Hülsbeck, V., Düffert, P., et al. Influence of Flow Rate and Different Size of Suction Cannulas on Splatter Contamination in Dentistry: Results of an Exploratory Study with a High-volume Evacuation System. Clinical Oral Investigations. 2022; 26(9): 5687-5696. https://doi.org/10.1007/s00784-022-04525-7
- Medical and First Aid. (1998, June 18). Occupational Safety and Health Administration. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.151