Lidocaine is a widely used local anesthetic in dentistry, known for its ability to successfully inhibit pain signals from sensory neurons, typically administered through topical creams or injections. Additionally, it has been found to have potential anticancer properties.1
Past research discovered lidocaine’s potential anticancer properties, yet the exact mechanism behind this discovery has remained elusive until now. A study explored the mechanism by which lidocaine triggers a cascade of cellular signals that lead to apoptosis (cell death) in head and neck squamous cell carcinoma (HNSCC), which could help improve cancer treatment outcomes.1
Lidocaine’s distinct bitter taste arises from its activation of a taste receptor known as T2R14, a receptor found in high concentrations in various cancer cells, particularly those in the head and neck. Researchers at the University of Pennsylvania postulated that lidocaine may interact with cancer cells through this receptor.1
“We were astonished to discover that lidocaine targets the very receptor that is most abundantly expressed in various types of cancer,” remarked Robert Lee, the study lead and assistant professor of Otorhinolaryngology Head and Neck Surgery at the University of Pennsylvania.2
The Study
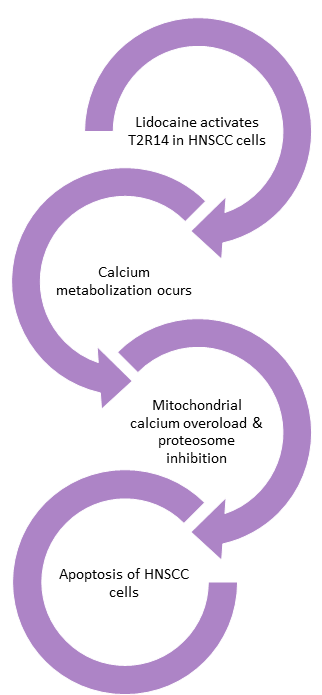
This study demonstrates that lidocaine triggers calcium responses in HNSCC cells through T2R14 receptors. It also shows that lidocaine reduces the viability of HNSCC cells and affects mitochondrial function by depolarizing the mitochondrial membrane potential, increasing mitochondrial reactive oxygen species (ROS), and inducing apoptosis.1
The apoptotic effect is blocked by specific T2R14 antagonists. However, it was found that lidocaine inhibits the proteasome in a T2R14-dependent manner, suggesting a new mechanism for T2R agonist-induced apoptosis and a connection between G-protein-coupled receptor (GPCR) signaling and proteasome inhibition.1
 The Results
The Results
The findings suggest that lidocaine could potentially be repurposed as a therapeutic agent for HNSCC, either through topical application or intratumor injection. Moreover, the study reveals that human papillomavirus (HPV)-associated HNSCCs have increased expression of TAS2R14, the gene encoding T2R14, indicating that HPV+ tumors might particularly benefit from T2R14 activation with lidocaine treatment.1
T2R agonists, including lidocaine, have been shown to disrupt HNSCC cellular function, mitochondrial membrane potential (MMP), and cell survival. The upregulation of TAS2R14 expression in some HNSCC cases suggests a potential personalized treatment strategy based on receptor expression. T2R receptors are found not only in the oral cavity but also in other epithelial regions affected by HNSCCs, making them accessible targets for treatment.1
With its high solubility, safety profile, and documented palliative effects, lidocaine could be easily incorporated into clinical practice as an injectable or topical anticancer therapy. This study also elucidates how lidocaine, unlike other anesthetics with similar sodium channel-blocking abilities, triggers apoptosis in certain cancer cells. It establishes a connection between lidocaine and endogenous T2R14 in HNSCC cells, suggesting that lidocaine’s effects are mediated through T2R14 activation, leading to calcium responses. Compared to other T2R agonists, lidocaine elicits the largest calcium responses, likely due to its potent and specific activation of T2R14 rather than sodium channel inhibition.1
“While we’re not claiming that lidocaine can cure cancer, we are excited about the possibility that it could enhance head and neck cancer treatment and advance the progress in improving treatment options for patients facing this challenging cancer type,” said Ryan Carey, an assistant professor and co-lead author of the study.2 Lidocaine holds promise as it can be conveniently injected near or around accessible oral tumors.1,2
Conclusion
“As a head and neck surgeon, we frequently use lidocaine,” Carey noted. “We know lidocaine is safe, we are comfortable using it, and it’s readily available, which means it could be seamlessly integrated into other aspects of head and neck cancer care.”2
The most prevalent head and neck cancer, squamous cell carcinoma, has a high mortality rate, with only 50 percent of patients surviving past five years, even with treatment. Thus, these results offer a glimmer of hope for individuals battling the disease.1,2
The implications of this research may extend beyond head and neck cancers, with the possibility that lidocaine could also benefit patients with other forms of cancer. A study published in the Journal of Clinical Oncology in April 2023 found that breast cancer survival rates increased when patients received lidocaine before surgery.2,3
Furthermore, lidocaine may not be the only drug capable of triggering cell death in cancers through this receptor. “What’s incredibly exciting is that a lot of existing drugs activate [T2R14], so there could be additional opportunities to consider repurposing other drugs that could safely target this receptor,” Lee added.2
The research team hopes that their proof of concept will pave the way for clinical trials exploring the addition of lidocaine to standard care therapy for patients battling head and neck cancer.
Before you leave, check out the Today’s RDH self-study CE courses. All courses are peer-reviewed and non-sponsored to focus solely on pure education. Click here now.
Listen to the Today’s RDH Dental Hygiene Podcast Below:
References
- Miller, Z.A., Mueller, A., Kim, T., et al. Lidocaine Induces Apoptosis in Head and Neck Squamous Cell Carcinoma Through Activation of Bitter Taste Receptor T2R14. Cell Reports. 2023; 42(12): 113437. https://doi.org/10.1016/j.celrep.2023.113437
- Dewan, P. (2023, November 23). Scientists Find Anesthetic Kills Cancer Cells via Unique Mechanism. Newsweek. https://www.newsweek.com/scientists-anesthetic-kills-cancer-unique-mechanism-1846521
- Badwe, R.A., Parmar, V., Nair, N., et al. Effect of Peritumoral Infiltration of Local Anesthetic Before Surgery on Survival in Early Breast Cancer. Journal of Clinical Oncology. 2023; 41(18): 3318-3328. https://doi.org/10.1200/JCO.22.01966