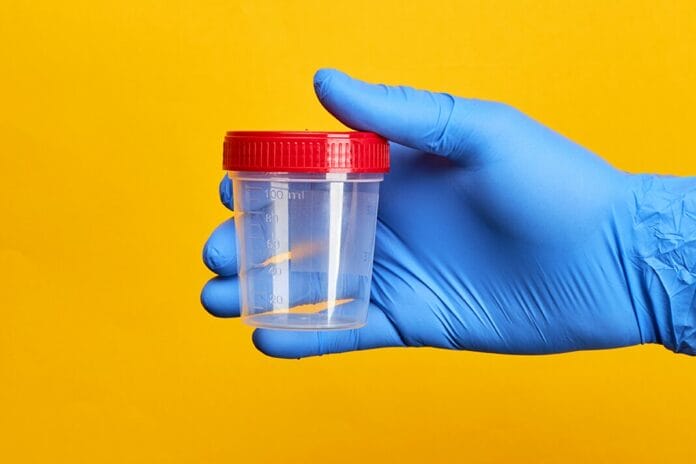
Researchers at the University of Michigan Health Rogel Cancer Center have created a urine-based test that detects minute DNA fragments shed by HPV-positive oropharyngeal squamous cell carcinoma. The initial findings of the study were published in JCI Insight. This novel approach holds the promise of better early detection of a cancer type currently lacking a dependable screening method.1
While human papillomavirus (HPV) is predominantly associated with cervical cancer, its role in contributing to oropharyngeal cancer is increasingly recognized.2
The urgency for early detection stems from the fact that identifying cancer at its earliest stages significantly improves patient outcomes.2
The Study
Utilizing whole genome sequencing, the research team found that tumor cells release ultra-short, less than 50 base pairs, cell-free tumor DNA (ctDNA) fragments. These fragments are transported from the bloodstream into urine via the kidneys. Conventional liquid biopsy tests, tailored to detect longer DNA fragments, are likely to overlook these small fragments, rendering them undetectable in urine or blood samples.1
The research team created a droplet digital PCR (ddPCR) assay designed to measure trans renal cell-free tumor DNA (TR-ctDNA) in urine, offering absolute quantification, greater precision, and higher throughput compared to next-generation sequencing (NGS).1
This assay was specifically developed for patients with HPV-positive oropharyngeal squamous cell carcinoma (OPSCC). In these patients, HPV DNA sequences circulate in the blood as ctDNA. HPV ctDNA is an ideal target for TR-ctDNA ddPCR assay development due to three key reasons: (a) 90% of HPV+ OPSCC patients share the HPV16 subtype, allowing a single HPV16 TR-ctDNA assay to be applicable to most patients; (b) HPV, being a nonhuman sequence, would result in a low background signal from individuals without HPV+ cancer; and (c) HPV16 can integrate at multiple sites within the tumor genome, producing a higher signal per tumor genome. Unlike HPV+ cervical cancer, where tumor DNA can be directly shed into the urine, HPV16 DNA in the urine of HPV+ OPSCC patients must be trans renal.1
The Results
The research team found that targeting ultrashort fragments was crucial for effective urine TR-ctDNA detection. Using the ultrashort amplicon assay, they achieved urine TR-ctDNA detection results that matched those from plasma ctDNA in HPV+ OPSCC patients. Additionally, through longitudinal urine samples from a small case series, the research team demonstrated proof of concept for the early detection of cancer recurrence.1
Therefore, these findings suggest that by focusing on ultrashort DNA fragments, TR-ctDNA detection becomes a feasible method for identifying HPV+ OPSCC and potentially monitoring cancer recurrence post-treatment.1
Co-first author Chandan Bhambhani, Ph.D., emphasized, “Our study underscores that traditional assays fail to identify ultrashort fragments present in urine, as they’re optimized for longer DNA strands. We’ve taken an unconventional path to engineer a urine test specifically targeting these elusive fragments.”2
Beyond head and neck cancer, the study outlines a methodology that could be adapted to detect other cancers. The test successfully identified ctDNA in urine samples from patients with breast cancer and acute myeloid leukemia, indicating broader applicability.1
Conclusion
Though still in the exploratory phase, this mail-in test has been disseminated for research to patients within a hundred-mile radius of Ann Arbor, Michigan. Participants submit a urine sample, which is then shipped back to the University of Michigan laboratory for analysis, enabling the detection of head and neck cancer.1
Moreover, the convenience of self-collection makes urine-based assays more appealing for post-treatment follow-ups compared to blood-based alternatives, potentially enhancing patient compliance.1
In conclusion, the research team advocated for the detection of ultrashort cancer-derived DNA fragments as essential to unlocking the full potential of TR-ctDNA analysis. This approach enhances noninvasive, sensitive, and widely accessible cancer detection and monitoring across diverse clinical applications.
Before you leave, check out the Today’s RDH self-study CE courses. All courses are peer-reviewed and non-sponsored to focus solely on high-quality education. Click here now.
Listen to the Today’s RDH Dental Hygiene Podcast Below:
References
- Bhambhani, C., Kang, Q., Hovelson, D.H., et al. ctDNA Transiting into Urine Is Ultrashort and Facilitates Noninvasive Liquid Biopsy of HPV+ Oropharyngeal Cancer. JCI Insight. 2024; 9(6): e177759. https://insight.jci.org/articles/view/177759
- Michigan Medicine – University of Michigan. (2024, April 16). Researchers Discover Urine-Based Test to Detect Head and Neck Cancer. ScienceDaily. www.sciencedaily.com/releases/2024/04/240416214642.htm